C−S-Selective Stille-Coupling Enables Stereodefined Alkene Synthesis
Jing Jing1, Ying Hu1, Zhenfeng Tian1, Yicheng Wang4, Liqin Yao3(姚丽琴)*, Lipeng Qiu4, Lutz Ackermann2, Konstantin Karaghiosoff5*, Jie Li1(李杰)*
1Key Laboratory of Organic Synthesis of Jiangsu Province, MOE Key Laboratory of Geriatric Diseases and Immunology, Suzhou Key Laboratory of Pathogen Bioscience and Anti-infective Medicine, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 215123 Suzhou, China
2Institut für Organische und Biomolekulare Chemie, Georg-August-Universität-Göttingen, Tammannstraße 2, 37077 Göttingen, Germany
3Yixing Traditional Chinese Medicine Hospital, 214200 Yixing, China
4School of Life Science and Health Engineering, Jiangnan university,214122, Wuxi, China
5Department Chemie, Ludwig-Maximilians-Universität München, Butenandtstr. 5–13, Haus F, 81377 Munich, Germany
Angew. Chem. Int. Ed. 2024, 63, e202408211
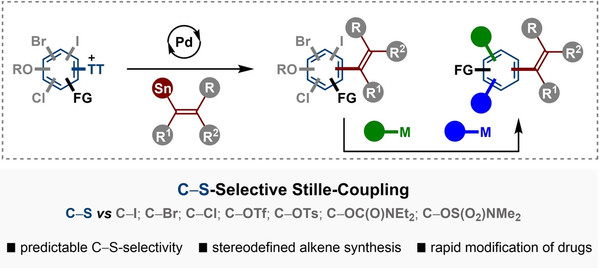
Abstract: A palladium-catalyzed highly C−S-selective Stille cross-coupling between aryl thianthrenium salts and tri- or tetrasubstituted alkenyl stannanes is described. Herein, critical challenges including site- and chemoselectivity control are well addressed through C−H thianthrenation and C−S alkenylation, thereby providing an expedient access to stereodefined tri- and tetrasubstituted alkenes in a stereoretentive fashion. Indeed, the palladium-catalyzed Stille-alkenylation of poly(pseudo)halogenated arenes displays privileged capability to differentiate C−S over C−I, C−Br, C−Cl bonds, as well as oxygen-based triflates (C−OTf), tosylates (C−OTs), carbamates and sulfamates under mild reaction conditions. Sequential and multiple cross-couplings via selective C−X functionalization should be widely applicable for increasing functional molecular complexity. Modular installation of stereospecific alkene motifs into pharmaceuticals illustrated the synthetic application of the present protocol in drug discovery.
Article information: //doi.org/10.1002/anie.202408211