Regulated Li+ Solvation via Competitive Coordination Mechanism of Organic Cations for High Voltage and Fast Charging Lithium Metal Batteries
Jiangtao Yu1, Xinyu Ma1, Xiuyang Zou2, Yin Hu1, Mingchen Yang1, Yuanli Cai1(蔡远利)*, Feng Yan1,3(严锋)*
1Jiangsu Engineering Laboratory of Novel Functional Polymeric Materials, Laboratory of Advanced Negative Carbon Technologies,Suzhou Key Laboratory of Soft Material and New Energy, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123, China
2School of Chemistry and Chemical Engineering, Huaiyin Normal University, No.111 West Changjiang Road, Huaian 223300, Jiangsu Province, China
3State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, China
Angew. Chem. Int. Ed. 2025, 64, e202416092
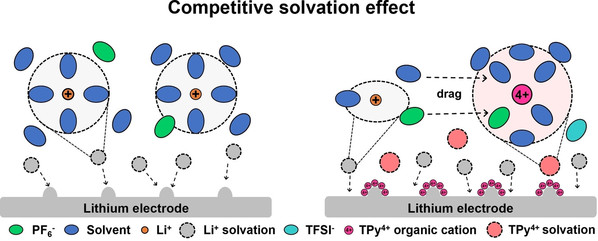
Abstract: Li+ solvation exerted a decisive effect on electrolyte physicochemical properties. Suitable tuning for Li+ solvation enabled batteries to achieve unexpected performance. Here, we introduced inert organic cations to compete with Li+ for combining electrolyte molecules to modulate Li+ coordination in the electrolyte. The relevance between the number of cationic sites in organic cations and the competitive solvation ability was explored. The organic cations with multiple cationic sites attracted solvent molecules and anions away from Li+ to form new solvated shell, improving the Li+ transport kinetics and desolvation process in electrolyte while enhancing electrolyte oxidation tolerance. Moreover, electrostatic shielding provided by organic cations and anion-derived robust SEI promoted uniform and rapid Li+ deposition on Li electrodes. With the positive effect of organic cations, Li||LiCoO2 (LCO) batteries showed high specific capacity (136.46 mAh g−1) at high charge/discharge rate (10 C). Furthermore, Li||LCO batteries exhibited good capacity retention (70 % after 500 cycles) at 4.6 V charge cut-off voltage. This work provides fresh insights for the optimization of electrolytes and battery performance.
Article information: //doi.org/10.1002/anie.202416092