Photocatalyzed Aryl C-H Fluorocarbonylation with CF2Br2
Kehan Zhou, Yuheng Xiao, Zhibin Huang, Yingsheng Zhao(赵应声)*
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China
Angew. Chem. Int. Ed. 2025, 64, e202414933
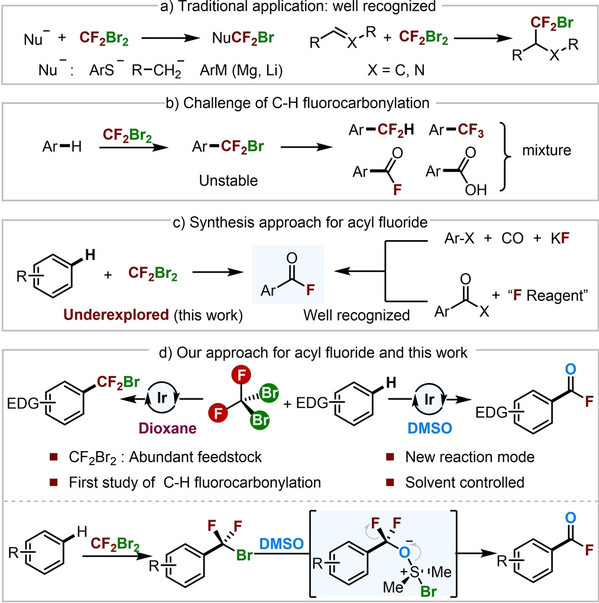
Abstract:The use of abundant and inexpensive fluorine feedstocks to synthesize fluorinated compounds is a promising strategy that has not been extensively investigated. Dibromodifluoromethane (CF2Br2) is an inexpensive fluorine source that has rarely been used for C−H fluoroalkylation. This study reveals an iridium-catalyzed, tunable strategy for synthesizing acyl fluorides and difluorobromomethylated products using CF2Br2. To achieve the desired products, this process only requires the change of solvent (from DMSO to 1,4-dioxane) under blue LED illumination. A variety of arenes and heteroarenes with electron-donating substituents were successfully used, yielding the corresponding products in moderate to good yields. Mechanistic experiments revealed that DMSO served a dual role, functioning as both solvent and nucleophilic reagent in C−H fluorocarbonylation.
Article information://onlinelibrary.wiley.com/doi/10.1002/anie.20241493