Investigation of Electron Transfer Properties on Silicalite‑1 Zeolitefor Potential Electrocatalytic Applications
Jin Yingying1, Yin Xichen1, Yu Guanghua1, Sun Qiming1,2(孙启明)*, Wang Jiong1,2(王炯)*
1Innovation Center for Chemical Science, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215006, P. R. China
2Jiangsu Key Laboratory of Advanced Negative Carbon Technologies, Soochow University, Suzhou 215123, P. R. China
J. Am. Chem. Soc. 2024, 146, 51, 35109–35116
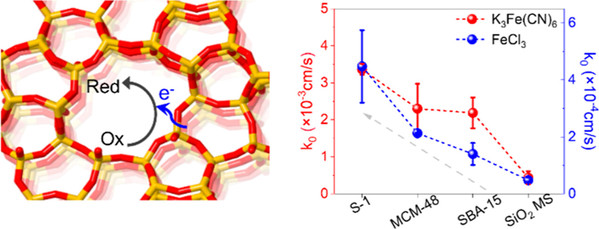
Abstract: To develop high-performance electrocatalysts is critical to sustainable conversion and storage of renewable energy. Silicalite-1 (S-1) zeolite is considered promising for constructing electrocatalysts featuring uniform and precise porosity and a stable structural skeleton even at extreme potentials. However, its electrochemical properties remain poorly understood, particularly regarding the roles of internal pore channels. Herein, inner- and outer-sphere electron transfer (ISET/OSET) routes on the S-1 zeolite were investigated by classical redox probes. The results for the first time revealed that the ISET kinetics inside the pores of S-1 zeolite is more rapid than that on external surfaces, optimized by microporous scale channels and terminated hydroxyl groups. Conversely, the kinetics of the OSET did not closely depend on the porosity and surface properties of the S-1 zeolite. These electrochemical insights further initiated a lithium-ion-incorporated S-1 zeolite with rapid ISET kinetics for electrocatalysis of oxygen reduction. It demonstrated a high performance of 85% selectivity for H2O2 production in a neutral solution and a yield of 9.2 mol gcat–1 h–1 when configured in a flow cell.
Article information: //pubs.acs.org/doi/10.1021/jacs.4c10258