Molecular architectures of iron complexes for oxygen reduction catalysis—Activity enhancement by hydroxide ions coupling
Poe Ei Phyu Win1, Jiahui Yang1, Shuwang Ning2, Xiang Huang3*(黄祥), Gengtao Fu2 , Qiming Sun1,4, Xing-Hua Xia5* (夏兴华), and Jiong Wang1,4*(王炯)
1Innovation Center for Chemical Science, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215006, China;
2Jiangsu Key Laboratory of New Power Batteries, Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing 210023, China;
3Department of Physics, Southern University of Science and Technology, Shenzhen 518055, China; 4Jiangsu Key Laboratory of Advanced Negative Carbon Technologies, Soochow University, Suzhou, Jiangsu 215123, China;
5State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China
PNAS, 2024, 121, e2316553121
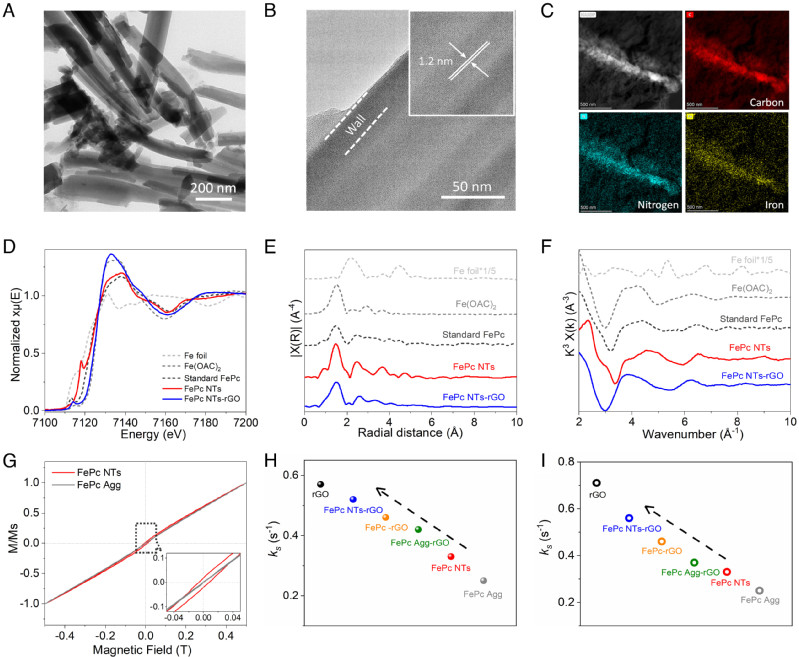
Abstract:Developing cost-effective and high-performance electrocatalysts for oxygen reduction reaction (ORR) is critical for clean energy generation. Here, we propose an approach to the synthesis of iron phthalocyanine nanotubes (FePc NTs) as a highly active and selective electrocatalyst for ORR. The performance is significantly superior to FePc in randomly aggregated and molecularly dispersed states, as well as the commercial Pt/C catalyst. When FePc NTs are anchored on graphene, the resulting architecture shifts the ORR potentials above the redox potentials of Fe2+/3+ sites. This does not obey the redox-mediated mechanism operative on conventional FePc with a Fe2+–N moiety serving as the active sites. Pourbaix analysis shows that the redox of Fe2+/3+ sites couples with HO− ions transfer, forming a HO–Fe3+–N moiety serving as the ORR active sites under the turnover condition. The chemisorption of ORR intermediates is appropriately weakened on the HO–Fe3+–N moiety compared to the Fe2+–N state and thus is intrinsically more ORR active.
Link: //www.pnas.org/doi/10.1073/pnas.2316553121